
The Electron
The electron is arguably the most famous of the elementary particles. They are the business end of the atom, and their dynamics give rise to virtually all of chemistry. They’re also the basic working particle of electricity, and their presence or absence in the silicon transistors correspond to the digital 0’s or 1’s of modern computers.Like all elementary particles, they have no known size. But they do have a known mass. It’s fairly small, at 0.511 MeV. The electron’s electric charge sets the standard for particle physics. With only a few rare exceptions, all known charges are multiples of the electrons electric charge, e.
Figure: The Electron's Charge
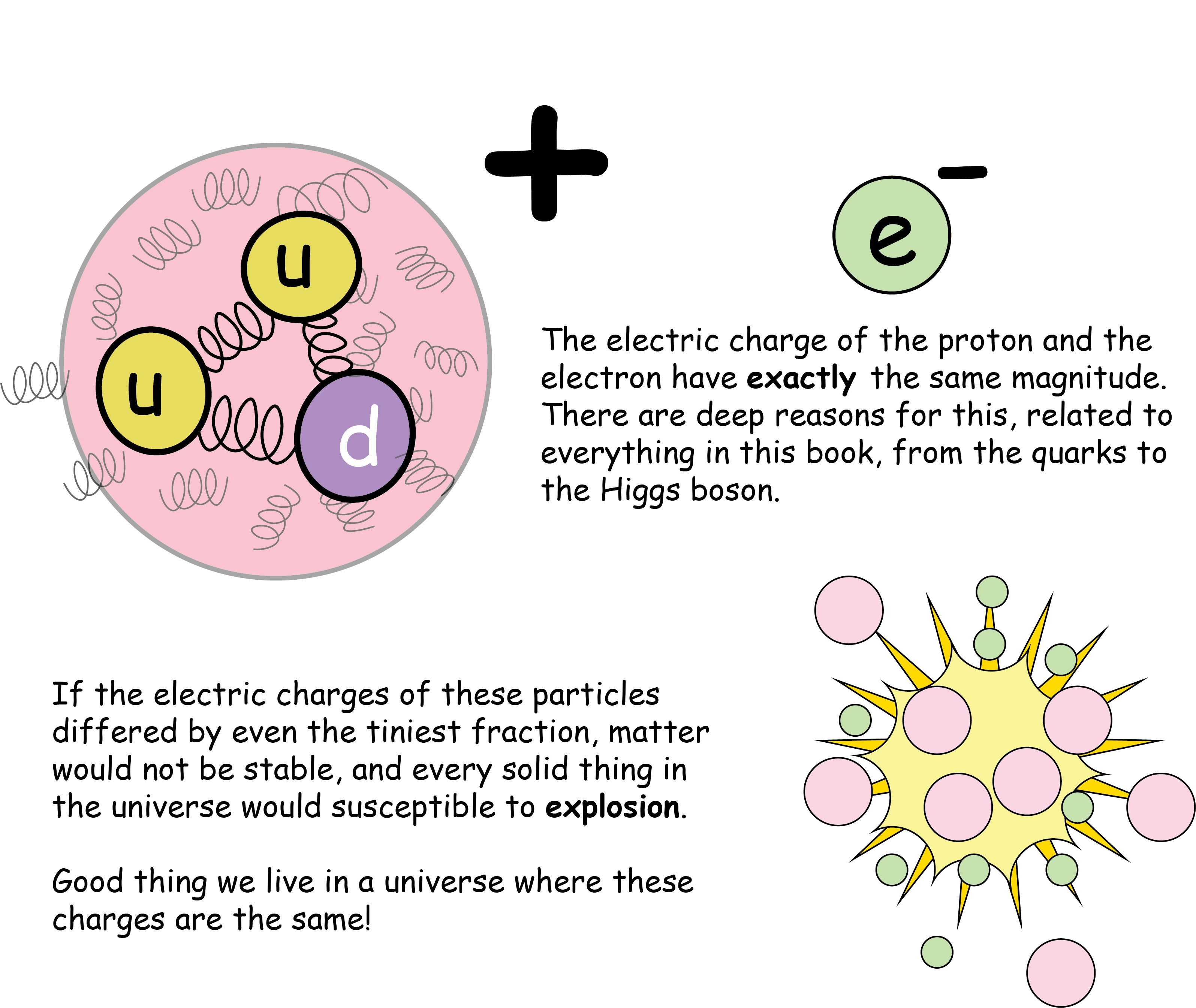
Atoms are electrically neutral because the electron's charge is identical to the proton's charge.
Electrons interact with other particles primarily via the electromagnetic force - photons - but they also feel the weak nuclear force and, of course gravitation.
So far as we can tell, electrons are stable particles. They don’t decay, and that’s good news! A decaying electron would either mean that a lighter particle with the same charge should exist - which we haven’t seen - or that electric charge would simply disappear. Any violation of the conservation of electric charge would contradict the very root of our understanding of how the forces of nature work.
The electron has an antiparticle partner - which looks almost identical except that its electric charge is positive. It’s common practice to call the anti electron a positron. If an electron and a positron meet, sometimes they’re orbit each other for a bit, but usually, they just annihilate into a pair of photons.
If there’s one particle in nature to study, it is definitely the electron.