The Neutron
With a mass of 939.565 MeV, the neutron is the second lightest baryon - that is, a particle made up of three quarks. The neutron's quarks include one up and two downs, so it is total electric charge is zero:2/3 e - 2x 1/3 e = 0.
The neutron may be electrically neutral, but don’t let that fool you, it still interacts with the electromagnetic field.Like the earth - and the proton - the neutron has a tiny magnetic field. It's a dipole field: with a north and south pole. Because moving electric charges generate magnetic fields, you might expect a spinning electric charge, like the proton, to have a magnetic field, but not the neutron. And yet it does.
What’s curious about the neutron’s magnetic field is that it’s so strong. It’s about 60% that of proton, although oppositely orientated. This fact was probably the first clue that the neutron wasn’t a fundamental particle, but was made up of some other junk.
The neutron is just a tiny bit heavier than the proton, which is fitting given that the down quark is just a tiny bit heavier than the up quark. Because the up and down quarks are related by the weak nuclear force, the neutron is unstable! Left to its own devices, one down quark will eventually decay to an up, emitting an electron and a neutrino in the process. This is all very hard to see, given that the neutron’s quarks swim in a bag of nuclear goo, but the net effect is that an electron sails away with a negative charge, leaving behind a positively charged proton.
Alone, the average life expectancy of a neutron is something like 15 minutes - which is an eternity by particle physics standards.
Figure: Neutron Decay
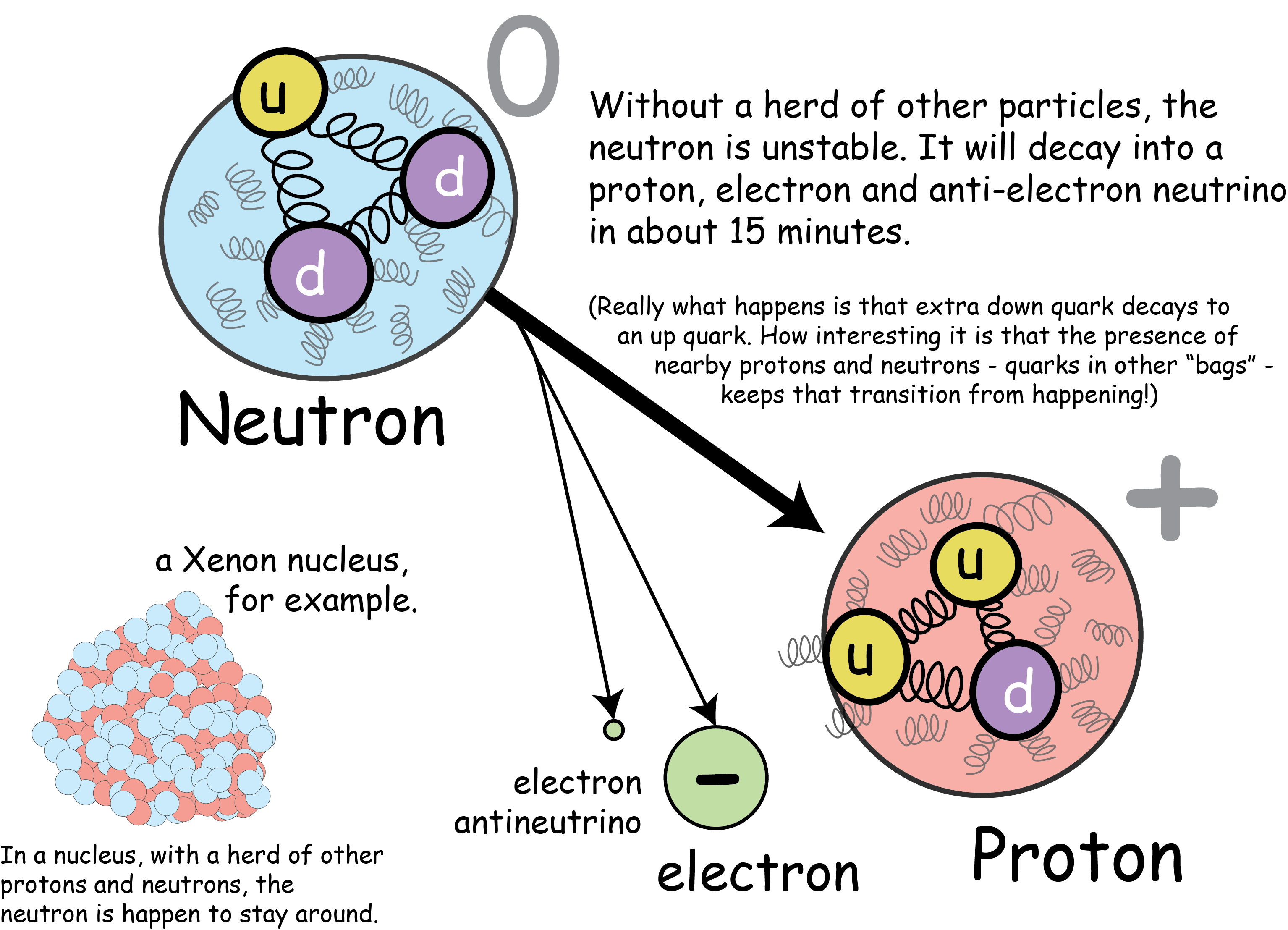
The neutron in isolation decays to the proton, which has a lower mass. The slightly heavier down quark converts to an up.
Given that a neutron decays in the time it takes to drink a cup of coffee, you might wonder why we have any neutrons left in the universe. Suffice it to say, neutrons are like cattle. When they’re herded together in a nucleus, they’re far more stable.
In fact, the number of neutrons goes up as the size of the nucleus does too. Helium-4 has two protons and two neutrons, and it’s pretty much stable. Iron-60 has 26 protons and 34 neutrons. It lives for millions of years. Uranium-238 has 92 protons and 146 neutrons. It lives for billions of years.
Unstable alone, yet stable in packs, the humble neutron is all around us, and makes up a good chunk of our mass, too.