The Electron Cloud
We are made up of molecules, and molecules are made up of atoms joined together by their electrons - or sometimes, by their lack thereof.Atoms are modeled by a nucleus surrounded by an electron cloud. We’ve discussed the nucleus earlier; it’s the hard center of the atom. It’s really, really small, as small as a speck of dust in the middle of a baseball stadium. Despite that fact, the nucleus makes up over 99% of the atom’s mass. Electrons are quite light by comparison.
The nucleus is small because the nucleons - those protons and neutrons - are bound so tightly together. The electron, by comparison, is barely hanging on. That’s why their orbits are so wide.
Because electrons are small and light, they are subject to the physics of quantum mechanics. This is a deep and important fact, but for practical purposes it means that the details of their motion around the nucleus are obscured. We can’t say with 100% certainty where the electron is, or how it is moving. And this obscurity is central to how atoms work.
In traditional models from classical physics, forces like electromagnetism causes charged particles to orbit each other, just like planets orbiting the sun. The size and shape of those orbits are related how much energy those plants have. And unlike planets, electrons are known to lose energy very, very quickly.
If electrons were really orbiting in circles or ellipses, the centrifugal force associated to that rotational motion would cause the electrons to lose a tremendous amount of energy. This would eventually collapse the atom, sending the electrons crashing into the nucleus. This paradox occupied was a big concern around the turn of the 20th century.
To understand why electrons don’t crash into the nucleus, it helps to consider a related mystery. Consider the simple case of the hydrogen atom: a single electron orbiting a single proton. The electron has no orbital angular momentum. How can an electron stay in orbit with a proton without any angular momentum?
Quantum mechanics provides a curious - if unsatisfying - resolution to this mystery. It obscures the true position of the electron. The electron is smeared out as a spherical shell around the nucleus. The electron isn’t really orbiting. It’s just smeared out, hanging in a particular region, which happens to surround the proton. Perhaps this explains the name, electron cloud.<
It’s important to understand that this obscurity isn’t just a failure of imagination or a failure of technology. You can prove it mathematically, and verify that proof experimentally. It’s not that we don’t know the details of the electron’s position and momentum, it’s as if those details simply don’t exist. They aren’t real. Weird as it is, it’s a literal fact of the universe that keeps the electrons bound to the proton.
There’s one other oddity that quantum mechanics bestows to the electron that’s important to the physics of atoms. It’s so important it has a name. It’s called the Exclusion Principle and it says that no two electrons can share the same configuration at the same time. That’s another deep statement with a lot of technical details, but its practical implications give rise to virtually all of chemistry.
Figure: Electron Cloud Orbitals
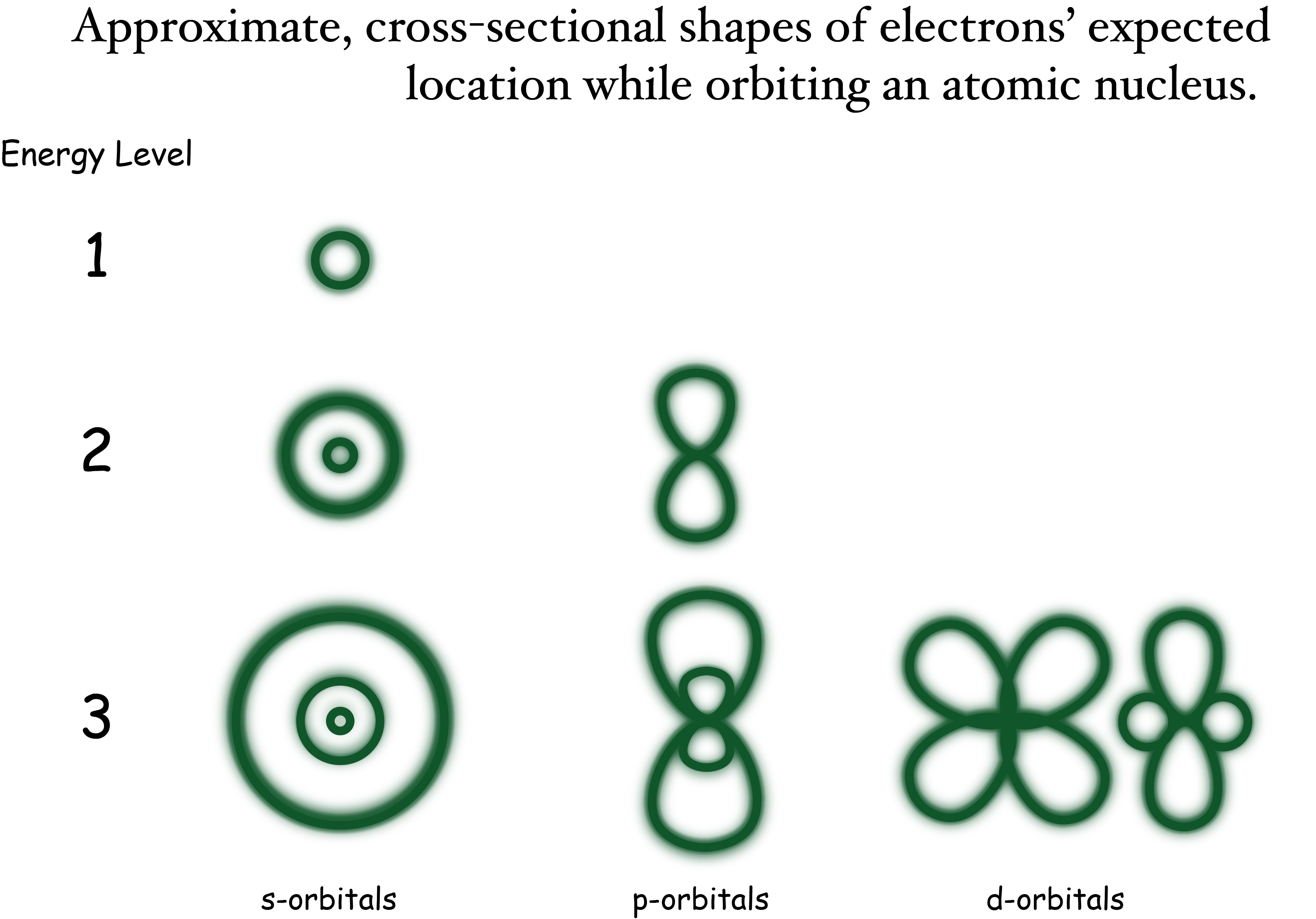
Quantum Mechanics constraints our ability to know with precision the electron's position in an atom. The best we can say is that the electron is constrained to be found 90% of the time in a number of geometric patterns called orbitals.
The exclusion principle means that each atom essentially has “slots” for individual electrons to fill. Typically, they fill in from lowest to highest energy. The more protons the nucleus has, the more slots for electrons the atom has to fill. Perhaps you learned about these slots in a chemistry class.
Higher energy slots have slightly different shapes. Some have angular momentum. Some do not. It is these complicated shapes that allow electrons form bonds between atoms. But this is as this particle physics series, chemical bonds must be another story, for another time.