The Atomic Nucleus
We are made of molecules, and molecules are made of atoms, and atoms are really, really small. Atoms are so small that its hard for our minds to comprehend it, but if you need a reference point take your height and divide it by a billion. Or maybe ten billion! Atoms are too small to rationalize.When we think about atoms, we model them as a nucleus surrounded by an electron cloud. Today, we are talking about that nucleus.
The nucleus is the hard center of the atom. It’s much, much smaller than the atom itself: about 100,000 times smaller. It's so small that if the atom were a baseball stadium, the nucleus is smaller than the baseball at the pitcher’s mound. Actually, the nucleus is more like a grain of clay atop of the pitcher’s mound.
Despite this small size, the nucleus makes up over 99% of the mass of the atom. So the nucleus is really, really small and really, really dense.
The nucleus is made up of smaller particles: protons and neutrons. Different atoms have different numbers of both, and they are all bound together by a strong force - the aptly named strong nuclear force - a force much stronger than the electromagnetic force that binds molecules together. In some sense the nucleus is so much smaller and denser than the electron cloud because the nuclear force is so much stronger that the electric force.
The chemical elements - familiar from the periodic table - are determined by the number of protons in a the nucleus of an atom. You can revisit the table and know immediately how many protons a given nucleus has. Hydrogen has one proton. Helium as two. Lithium has three. Carbon has six. Silicon has fourteen, and Iron has twenty-six protons.
Figure: Helium Isotopes
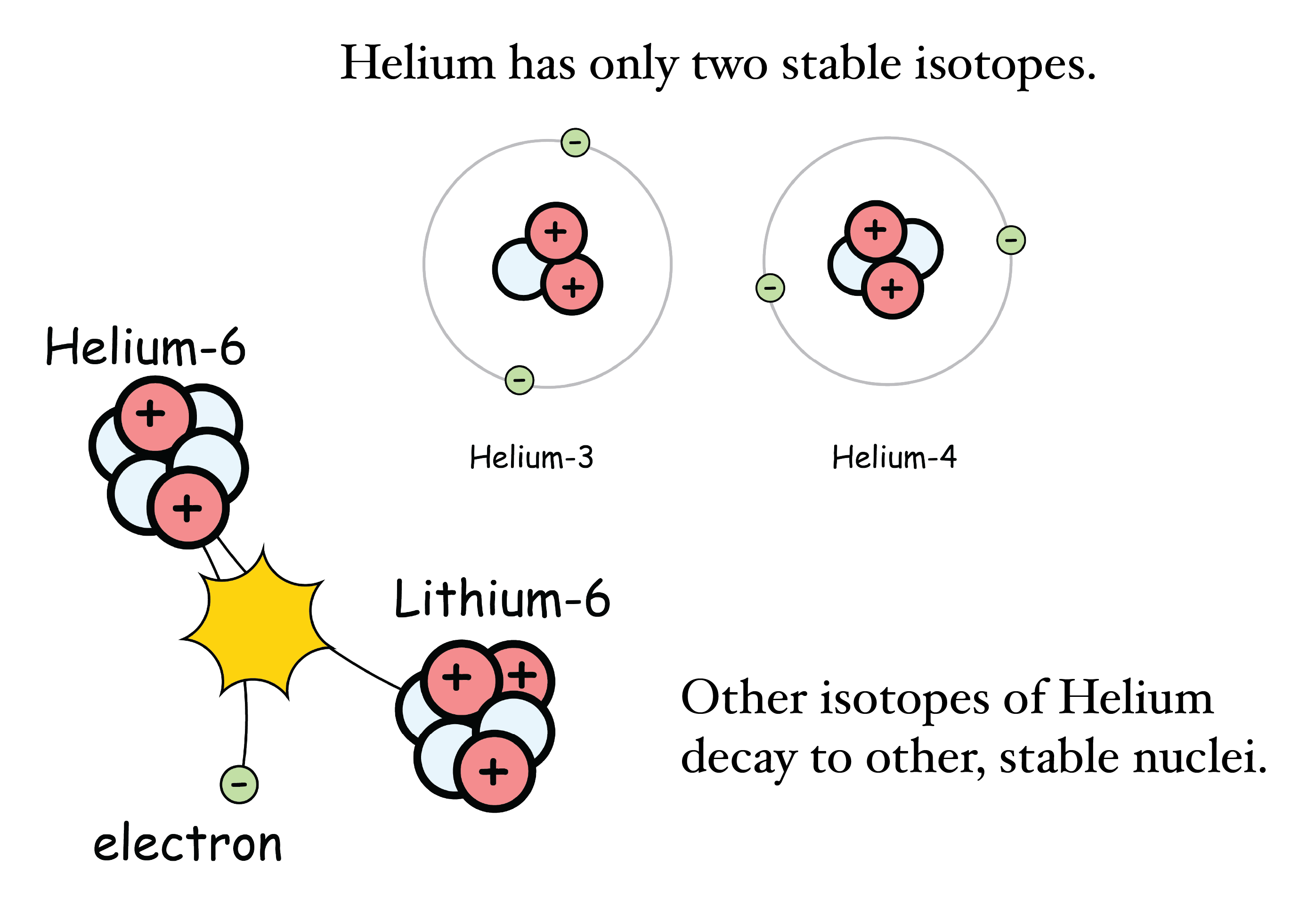
The same element can have different kinds of nuclei, that is, different isotopes. Helium has a number of variants, each with different numbers of neutrons. Above two neutrons, Helium is unstable to nuclear decay.
Atoms of the same element might have different numbers of neutrons. Some numbers are more common than others. Part of this is how those nuclei were created and part of this is stability. For example, you'll never see a hydrogen atom with twelve neutrons. It's just not stable. Unstable nuclei are difficult to form and spontaneously break apart. A nucleus might spit out some particles, or it might even suck in others. On exceptional occasions, a nucleus fracture into multiple pieces. The details are manifold and make up the entire field of nuclear physics. Nuclear physics has given us a chart of which nuclei are known and which are stable. Each has a story, but typically the number of neutrons in a nucleus increases with the number of protons. We’ll be sure to share a few of these stories with you in this series.